All Assay Types Descri-bed in Ph. EUR and USP in One Software
The PLA 3.0 software supports the analysis of all types of biological assays and provides a range of biostatisti-cal methods for immunoassays, ELISA, and more. Our PLA 3.0 all-in-one software bundle provides the Biologi-cal Assay Package and the Dose-Response Analysis Package.
The current version PLA 3.0 is fully compliant with GAMP and 21 CFR Part 11. PLA is the most commonly used software for biostatistical analysis in highly regu-lated pharmaceutical and biotech industries.
Building an Assay Archive for Later Review
Over the months and years, you will spend considerable amounts of time and money on your assays. With PLA 3.0, you make sure that all your assays remain acces-sible for later review. PLA 3.0 keeps them in a database, ready to be retrieved with just a few clicks.
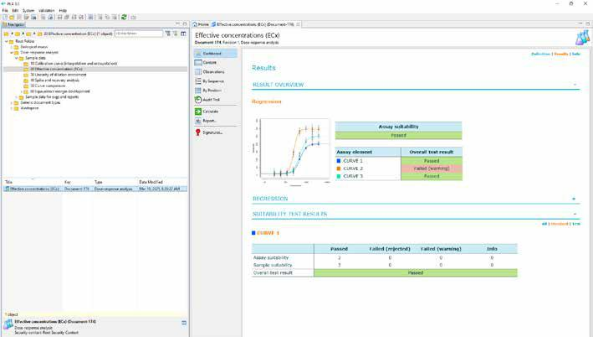
FDA Compliant, User-friendly Framework
Compliance with FDA 21 CFR Part 11 is one of the most important requirements for software meant for regulated environments. The challenge is to make the software fully compliant at the core and user-friendly on the surface. With PLA 3.0, we managed to achieve exactly that.
Covering the Entire Lifecycle of Your Product
With PLA 3.0, you can use the same software for the entire project - from development all the way through to production. The software will adapt to the stage of the project. We built several features into PLA 3.0 that make it not just suitable but in fact ideal for projects and teams of virtually any size.
Going Beyond Service: Your PLA Support Contract
The PLA Support Contract helps you get the most out of PLA. The first year is included in every purchase. We provide a support portal for any issue around PLA and have a team of biostatisticians and (bio)chemists ready to support you.
Ready to Integrate With What You Already Use
PLA 3.0 is a highly versatile framework, ready to be extended to suit your particular needs. We developed a number of modules that allow you to connect PLA 3.0 to other programs or equipment, such as plate readers. In effect, your bioassay analysis will be independent of the device you are using to acquire the measurement values.
Users of PLA 2.0/2.1: Prepare to Reach the Next Level
When we published PLA 2.0 and 2.1, these programs were the most advanced we had developed so far.
And it’s the same with PLA 3.0. So it does make a lot of sense to switch from these previous versions to the newest one. You will reach the next level of bioassay analysis.
PLA 3.0 UNDER THE HOOD
Intuitive basic concept: Handle data as documents
PLA 3.0 is a software framework for biostatistical analysis in GxP and non-GxP en-vironments. The architecture of PLA 3.0 provides all the functionality required for regulated environments through the framework, while all statistical functionalities are delivered as document package add-ons.
Easily extensible framework
DOCUMENT CONCEPT
The basic idea is the concept of electronic documents, which we derived directly from the requirements of the FDA 21 CFR part 11 regulation. All data and meta data is kept in one single information unit - the document. The structure and capabilities of a document are defined in the document packages.
The framework protects these documents with electronic and digital signatures ac-cording to the 21 CFR part 11 regulation. To give you greater flexibility, documents are organized in folders where several restrictions can be applied (permissions, privileges, mandatory templates).
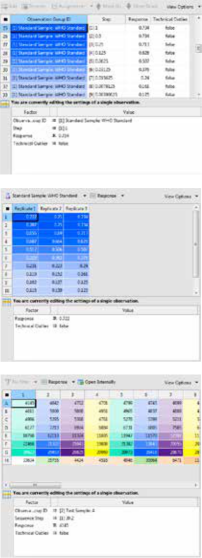
DOCUMENT PACKAGES
A document package contains one or more document types and calculation routines as well as required report templates and data for operational qualification (OQ). The PLA 3.0 base system comes with the
Elastic Forms Editor
The Elastic Forms Editor is the central editor of PLA 3.0. It allows you to edit docu-ments of any complexity in a fast, easy and efficient way. Context sensitive help for every field supports you with valuable information. The built-in template support lets you create and protect powerful templates to simplify usage and to support efficient standard operating procedures.
Data Editors
The system comes with three data editor perspectives. They allow different views on your data sets. You, as the user, can decide whether it is more efficient to view or edit data points line by line, or per sample along a dilution series or in a posi-tion factor perspective. Powerful tools like color-coding of fields help you to prevent input errors.
Reporting
Correct and validated reporting is critical in controlled environments. The PLA sys-tem supports validated reports to ensure a valid and trustable reporting of results.
DOSE-RESPONSE ANALYSE PACKAGE
The Dose-Response Analysis Package provides various biostatistical methods for im-munoassays, ELISA, and more.
The package features several equivalence test and development options for calibra-tion curves. You can employ equivalence tests for parameter estimates, point estimate tests for parameter estimates, and ANOVA terms. Regarding equivalence margin de-velopment you can use your historic assay runs to develop acceptance criteria. The package provides test strategy development, visualizations, and simulations.
The biostatistical methods include:
INTERPOLATION ON CALIBRATION CURVES
Interpolation analysis allows you to plot the observation data of the Standard sample and the Test samples, calculate the standard curve, and determine the unknown con-centration by interpolation on this curve. Statistics for interpolated results and dose-response curve characterization include averages, standard deviations, coefficient of variations, and recovery rates. Visualize results in overlay plots.
SPIKE-AND-RECOVERY ANALYSIS
Spike your test samples with different concentrations of your analyte to identify matrix effects or determine the precision of your assay within the assay range.

LINEARITY-OF-DILUTION ASSESSMENT
Determine the precision of your method at different levels of dilution.
EFFECTIVE-CONCENTRATION CALCULATION
Calculate the effective concentration of your sample in the concentrations you require by adding additional calculations.
CURVE COMPARISONS
Individually plot test samples and use the document dashboard or one of the document reports to compare the resulting curves. We support various analytical (regression) models.
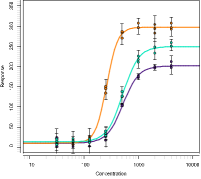
ENHANCED RESPONSE DATA PROCESSING
The package also features enhanced response data processing. You can adjust and normalize data either by a fixed value or by another assay element. Use these features, for example, to subtract the absorbance of blank wells or to divide response values by the mean absorbance of maximum binding wells. You can also employ transforma-tions–logarithmic, square, and square-root. Moreover, you can average your replicates.
BIOLOGICAL ASSAY PACKAGE
PLA 3.0 supports all types of biological assays according to European Pharmacopoeia, Chapter 5.3 and US Pharmacopeia <111>, <1032>, <1033>, <1034>: Quantitative Response Assays
(Parallel-Line, Parallel-Logistic, Slope-Ratio) and Dichotomous Assays (Quantal Re-sponse, Binary Assays). PLA 3.0 also supports all different weighting methods for combination calculations and the automatic data aggregation of independent assay data. Additional document types are available for Equivalence Margin Development, Control Charts and a Basic Bioassay Protocol.
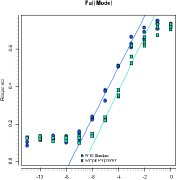
PARALLEL-LINE ASSAY
A parallel-line assay is the classical method to calculate a relative potency for a dilution assay. It is a linear fit which covers only the (near-) linear portion of the dose-response relationship without their asymptotes.
PLA 3.0 supports parallel line assays along with additional functionality to deter-mine acceptable regions of the dose-response curve (configuration optimization). The parallel-line method is a robust analytical method based on D.J. Finneys Statis-tical Methods in Biological Assay, 3. edition, 1978. It is supported by the European Pharmacopoeia and the US Pharmacopeia.
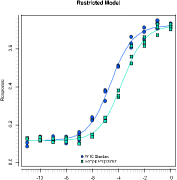
NONLINEAR QUANTITATIVE RESPONSE ASSAY
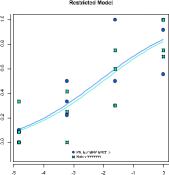
A nonlinear quantitative response assay (also known as a parallel-logistic assay) is a full curve fit method which takes the whole dose-response relationship into con-sideration, including asymptotes. PLA 3.0 supports different types of nonlinear full curve fits. The 4-parameter logistic curve fit is the most common approach. It models a symmetric sigmoidal dose-response correlationship. The 5-parameter logistic fit function adds an asymmetry parameter. The 3-parameter models is a reduced 4-pa-rameter model, where one of the asymptotes has to be set to a fixed value or to the mean of a control line, which allows the system to deal with truncated data.
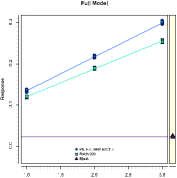
SLOPE-RATIO ASSAY
In contrast to parallel-line assays or nonlinear quantitative response assays, slope-ratio assays are carried out on a linear dose axis. They are most commonly used in microbiological applications.
The linear regressions of the standard and the sample intersect at zero dose. The potency is then calculated from the ratio of the slopes, the confidence intervals of the potencies are calculated using Fieller’s Theorem.
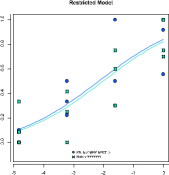
DICHOTOMOUS ASSAY
Dichotomous Assays (which are also called quantal response assays or binary assays) are assays based on binary outcome, i.e. a number of specimens shows a given re-sponse at a specific dose of an active ingredient. The assays are usually analyzed using the probit- or logit-method. PLA 3.0 supports both of these functions.
STATISTICAL TOOLS
Flexible Assay setup
The assay setup in PLA 3.0 is very flexible. You can setup any assay system with a free number of test or control samples and control lines as well as free numbers of replicates and dilution steps. Symmetric and asymmetric assay setups are sup-ported. When used in combination with the template system of PLA, any assay con-figuation can be set up in a GxP compliant way.
Outlier Detection
PLA 3.0 supports four optional outlier detection methods: Dixon Test, Grubb’s Test, Studentized Residual and a test based on the standard deviation of the treatments. Configuration Optimization
While the parallel-logistic assay (full curve fit) describes the whole dose-response correlation, parallel-line assays focus on the significant part of the dose-response relationship. PLA 3.0 is able to locate the significant parts of the dose-response cor-relation automatically to determine the optimal assay configuration.
Curve Fitting
In quantitative response assays both parallel-line assays and parallel-logistic assays (3–, 4– and 5-parameter sigmoidal functions) are implemented. Transformation func-tions for the response values are available for all models to reduce heteroscedasticity. The curve fitting of PLA 3.0 has been extensively optimized to provide high-quality curve fits, since bioassays tend to be very sensitive to the slightest deviations.
SOPHISTICATED TEST SYSTEM
Testing can be done either by difference/hypothesis testing, as described in the European Pharmacopoeia, or by similarity/equivalence testing, which was intro-duced by the US Pharmacopeia chapters <111>, <1032>, <1033> and <1034>. The test system of PLA 3.0 is very flexible and lets you perform a number of different tests, which you can configure exactly according to your needs.
For example, PLA 3.0 can test for potency, both relative and absolute. In addition, you can define potency ranges and confidence intervals. You can even run a test based on the number of outliers.
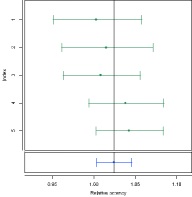
COMBINATION OF ASSAY RESULTS
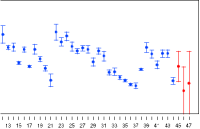
Combinations of assay results are not just means of potencies. To get optimal results, you need to consider the confidence intervals. PLA supports all methods of combina-tion described by the European Pharmacopoeia and the US Pharmacopeia. PLA also supports all different weighting methods for combination calculations and automatic data aggregation of independent assay data.
Combined relative potency confidence interval compared to the input confi-dence intervals
BASIC BIOASSAY PROTOCOL
The Basic Bioassay Protocol is a powerful document type to implement the most common workflow in bioassay analysis: combining the results of multiple indepen-dent assay runs into reportable values.
The Basic Bioassay Protocol controls all required steps: the assay is defined in the Basic Bioassay Protocol, which automatically creates a set of linked Quantitative Response Assays and Combination of Assay Results Documents. The results are au-tomatically and safely transferred between the Quantitative Response Documents and the Combination Calculation – so no retyping is required.
Finally, the Basic Bioassay Protocol is capable of handling retests. Just raise the number of your assay replicates and the Basic Bioassay Protocol will add the re-quired number of runs. This can be handled per assay run, but there is also an option to control this on a per sample level in multi sample assays.
EQUIVALENCE MARGIN DEVELOPMENT
The development of equivalence margins for use according to the US Pharmacopeia is a challenging task. PLA supports you with development assays which are used to develop the margins and verification assays that verify your test strategies. Test strategies are selections of available predefined or calculated margins that offer the possibility to include different strategies. The strategies can be visualized. The system is going to simulate a certain number of acceptable assays for every defined strategy to support the visual verification of the strategies.Besides this, the equiva-lence margin development offers a broad variety of equivalence tests.
At the end stands the development report that gives you many insights into the development process and results like for example detailed plots of the input assays.
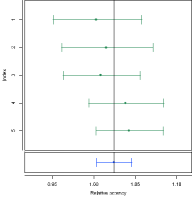
Visualization of Equivalence Margin Development
CONTROL CHARTS / MONITORING
The Control Chart document type in PLA 3.0 allows the trending of different param-eters of your biological assay. Define any number of trending parameters and differ-ent limits to keep your assay under control.
You can use the Control Chart document in two ways. You can edit or import data from any source, allowing to use the trend chart universally. Or you can directly aggregate the corresponding data from your calculation results as required in regu-lated environments.
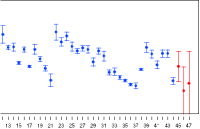
Control Chart
COVERING THE ENTIRE LIFECYCLE
In order to use software in companies or teams of different sizes, it should be easily extensible and scaleable. We therefore built several features into PLA 3.0 that make it not just suitable but in fact ideal for projects and teams of virtually any size.
TEMPLATES
PLA 3.0 comes with a new template engine. Templates can be defined by authorized users. They decide about the visibility, access level and default values in a docu-ment. Templates can be signed electronically and the administrator can define the mandatory use of specific templates in the database or in database sections. The template engine was designed to support the easy implementation of standard op-erating procedures (SOPs) within PLA.
VALIDATION AND
GXP COMPLIANCE
According to GAMP software has to be validated on the customer’s computer system. The software vendor is only able to verify the software in his labs. The optional Validation Package helps you to manage the tasks of installation qualifica-tion, operational qualification and perfor-mance qualification (IQ, OQ, PQ) fast and efficiently.
 |
INTEGRATION
PLA has a full set of interfaces for the import of raw data from data acquisition systems, for the export of data to e.g. documentation systems and for the reporting into many target systems. Individual modules can be created at low cost.
Import Modules are now distinguished into Document Import Modules creating a complete document with all settings and Data Acquisition Modules. Data Acquisi-tion Modules are able to import data into the data table of any document type. (Note: They replace the former PLA 2.x Import Modules.)
Document Import Modules
Document Import Modules generate fully specified documents of a specific type. They are useful when an external format contains every information required to create a specific document. E.g. if a third party program deals with biological assays a Document Import Module can be set up in a way where a completely specified quantitative response assay is delivered. In this case the Document Import Module converts all settings and creates the appropriate PLA 3.0 document.
Data Acquisition Modules
Data Acquisition Modules are used to connect external systems directly to the data tables of a PLA 3.0 document. Typical examples are Data Acquisition Modules for plate readers. A plate reader delivers a stream of measurement values that need to be imported to the data table of a specific document in a GLP/GMP compliant way. Data Acquisition Modules are the most common import modules used by PLA. Cur-rently, we have many different Data Acquisition modules available.
Data Acquisition Modules are licensed by the supported external format. They are not specific to the target document type.
Document Export Modules
Export Modules are available to export PLA 3.0 Document to a specific External For-mat. They export the PLA 3.0 Document and transform them into the target format. They can be used to connect PLA to other external systems (e.g. LIMS systems).
TRANSFER OF DATA AND TEMPLATES
PLA allows to transfer data and templates between projects, sites and companies in a secure manner.
The trustability and integrity of the data is assured by a combination of electronic signatures, that are preserved in the transfer, and cryptographically secured data transfers. PLA secures the information with the help of its own integrated PKI (Pub-lic Key Infrastructure). This operation is completely transparent for the user. It as-sures the secure communication of data and templates to different projects, sites or CROs.
ROLES AND PERMISSIONS
PLA 3.0 comes with an updated sophisticated permission system scalable from single seat to global installations. Typical roles are predefined in the system but can be altered to match your companies needs. All the settings are combined together in named security contexts that can easily be applied to new folders.
PLA Content Editor
21 CFR PART 11 COMPLIANCE
ADVANCED SECURITY FEATURES
In accordance with the FDA 21 CFR part 11 PLA has its own security infrastructure that requires users to log into the system. User accounts and their roles are defined with an easy-to-use interface. The accounts and their roles are database specific.
In addition to this account management PLA is fitted with the full range of security options required by the 21 CFR part 11. The PLA Administrator can define secu-rity policies for each database in accordance to regulatory or your company’s need. The feature includes password complexity, password aging, password blocking and password history rules. You may also define inactivity locks to prevent unauthorized access to the system.
ELECTRONIC SIGNATURES
Electronic signatures can be applied to all documents in PLA. The application of electronic signatures is a requirement of the 21 CFR part 11. With PLA’s advanced data storage technology electronic signatures can even be moved between different installations of PLA (e.g. between your CRO and your company).
DATA TRACEABILITY / AUDIT TRAIL
PLA has its own audit trail that covers all changes of data and properties of your documents and of all security features inside PLA. The audit trail can be inspected on a per-database and a per-document level. Filter and export functions have now been included into the base features of PLA 3.0. Acquired raw data can always be traced back to its original import source.
DIGITALLY SIGNED ELECTRONIC RECORDS
PLA benefits from the XML industry standard for the storage of electronic records. This very flexible format has the main advantage that it is human readable, which is another requirement for compliance.
PLA makes use of the XML Signature 1.0 Industry standard to assure the integrity of all the data that PLA works with. The XML Signature Standard applies a digital cryptographic signature to each data package. With the help of this signature the integrity of the electronic records is checked every time PLA makes use of them. This integrity check prevents any unauthorized or unwanted data modification, e.g. by computer defects.
For over 20 years, Stegmann Systems has been developing their software solution PLA which supports the develop-ment, execution and analysis of biological assays.
PLA is used by over 700 companies worldwide. Among them are the top 100 pharmaceutical and biotech companies together with laboratories, universities, research institutions and regulatory authorities.
The current version of PLA 3.0 supports the analysis of all types of biological assays and provides a range of biostatisti-cal methods for immunoassays, ELISA, and more. Our PLA 3.0 all-in-one software bundle provides the Biological Assay Package, Dose-Response Analysis Package and a fully compliant framework.
The established and well known Biological Assay Package supports all types of biological assays according to European Pharmacopoeia, Chapter 5.3 and US Pharmacopeia <111>, <1032>, <1033>, <1034>: Quantitative response assays (parallel-line, parallel-logistic, slope-ratio) and dichotomous assays (quantal response, binary assays). PLA 3.0 also sup-ports all different weighting methods for combination calculations and the automatic data aggregation of independent assay data. Additional document types are available for equivalence margin development, control charts and a Basic bioassay protocol.
The Dose-Response Analysis Package provides biostatistical methods, equivalence testing and equivalence margin development for calibration curves, enhanced data processing, and subgroup analysis. The methods support linearity-of-dilution assessment, spike-and-recovery analysis, effective-concentration calculation, and curve comparisons. Docu-ment reports and dashboards add to its value and usability.
The current version PLA 3.0 is fully compliant with GAMP and 21 CFR Part 11. PLA is the most commonly used software for biostatistical analysis in highly regulated pharmaceutical and biotech industries.
© Copyright 2000-2025 COGITO SOFTWARE CO.,LTD. All rights reserved